Abstract
The effective synthesis of two-dimensional transition metal dichalcogenides alloy is essential for successful application in electronic and optical devices based on a tunable band gap. Here we show a synthesis process for Mo1−xWxS2 alloy using sulfurization of super-cycle atomic layer deposition Mo1−xWxOy. Various spectroscopic and microscopic results indicate that the synthesized Mo1−xWxS2 alloys have complete mixing of Mo and W atoms and tunable band gap by systematically controlled composition and layer number. Based on this, we synthesize a vertically composition-controlled (VCC) Mo1−xWxS2 multilayer using five continuous super-cycles with different cycle ratios for each super-cycle. Angle-resolved X-ray photoemission spectroscopy, Raman and ultraviolet–visible spectrophotometer results reveal that a VCC Mo1−xWxS2 multilayer has different vertical composition and broadband light absorption with strong interlayer coupling within a VCC Mo1−xWxS2 multilayer. Further, we demonstrate that a VCC Mo1−xWxS2 multilayer photodetector generates three to four times greater photocurrent than MoS2- and WS2-based devices, owing to the broadband light absorption.
Similar content being viewed by others
Introduction
The band gap modulation of two-dimensional (2D) transition metal dichalcogenides (TMDCs) has been intensively studied, because of their various applications in optoelectronic devices such as photodiodes, phototransistors and solar cells1,2,3. It is well known that the band gap of 2D TMDCs is dependent on the number of layers4,5,6. In addition, alloying 2D TMDCs through the synthesis of Mo1−xWxS2, Mo1−xWxSe2 or MoS2xSe2(1−x), for example, is another way of practically modulating the band gap. This is an effective approach because of the good thermodynamic stability at room temperature of the alloys, as predicted by theoretical calculations7,8,9,10,11,12,13,14. Furthermore, recent studies have shown that a vertically composition-controlled (VCC) 2D TMDCs multilayer is feasible for the high performance optoelectronic devices due to functionality of interlayer such as interlayer transition15,16,17,18,19,20,21,22. However, the reported synthesis processes for 2D TMDCs alloy and VCC 2D TMDCs multilayer, such as exfoliation, chemical vapor deposition and transfer, are limited in respect of systematic control of the composition and the number of layers, and clean interface for strong interlayer coupling9,11,21,23. Hence, an improved synthesis process for 2D TMDCs alloy and VCC 2D TMDCs multilayer is highly required.
Atomic layer deposition (ALD), which is based on surface reactions between precursors and reactants, has benefits such as high purity, thickness control on the atomic scale and large area uniformity24,25. In particular, it is suitable for the synthesis of alloy thin films with precisely controlled composition using the super-cycle method26,27,28. In addition, a continuous super-cycle process with different cycle ratios can produce a VCC multilayer with a clean interface29. In a previous report, we have shown that atomically thin, layer-controlled and wafer-level uniform 2D WS2 can be synthesized by sulfurization of ALD WO3 thin films30.
Here we report a synthesis method of Mo1−xWxS2 alloys by sulfurization of super-cycle ALD Mo1−xWxOy alloy thin films. Using this method, we systematically control the composition and layer number (from mono- to tri-layers) of Mo1−xWxS2 alloys by controlling the cycle ratio between the ALD MoOx and WO3. The bandgaps of the Mo1−xWxS2 alloys are precisely controlled as functions of the composition and layer numbers of each respective alloy, as measured based on the photoluminescence (PL) spectra. Scanning transmission electron microscopy (STEM) shows the mixing of Mo and W atoms with shared metal atom sites in monolayer Mo1−xWxS2 alloy. Furthermore, we develop a process to synthesize a VCC Mo1−xWxS2 multilayer using a sequential super-cycle ALD process—specifically, 5 continuous super-cycles of ALD with different cycle ratios for each super-cycle. Ultraviolet–visible spectrophotometer analysis shows that the synthesized VCC Mo1−xWxS2 multilayer has stronger interlayer coupling than that of a stacked VCC Mo1−xWxS2 multilayer fabricated by the individual transfer of each monolayer Mo1−xWxS2 alloy. This can be attributed to the clean interface between each layer in the synthesized sample15,21.
Results
MoS2 synthesis
Previously, we reported the synthesis of WS2 using sulfurization of ALD WO3 thin film with a one-step sulfurization process at 1,000 °C (ref. 30). These synthesized WS2 exhibit smooth and continuous surfaces with layer controllability from mono- to tetra-layer. Based on this result, we sulfurized ALD MoOx thin film (nine cycles, optimization of ALD MoOx is represented in Supplementary Fig. 1) using a one-step sulfurization process at 1,000 °C (see experimental section) to synthesize MoS2. Figure 1a,b comprises scanning electron microscope (SEM) and atomic force microscopy (AFM) images of sulfurized MoOx thin film using the one-step sulfurization process at 1,000 °C. In contrast to WS2, however, the MoS2 shows a rough and non-continuous surface, and the measured root mean square (r.m.s.) is much larger (1.4 nm) than that of the SiO2 substrate (0.37 nm). We surmise that this discrepancy between the MoOx and WO3 thin films sulfurized at the same temperature (1,000 °C) is caused by the relatively lower vaporization temperature of MoOx (∼700 °C) in comparison with WO3 (over 1,100 °C)31. In other words, the MoOx is vaporized before the conversion to MoS2 is complete, resulting in a rough surface.
(a) SEM and (b) AFM images of sulfurized MoOx thin film using one-step sulfurization process at 1,000 °C. SEM and AFM images of sulfurized MoOx thin film using two-step sulfurization process with first-sulfurization temperatures of (c,d) 600 and (e,f) 800 °C, respectively. Scale bars, (a,c,e) 200 nm and (b,d,f) 0.5 μm.
Therefore, we examined the effect of sulfurization temperature on the roughness of the sulfurized ALD MoOx thin film. To achieve this, we conducted a two-step sulfurization process, which consists of a low-temperature first step for the sulfurization of the MoOx and a high-temperature second step to enhance the MoS2 crystallinity. The first-sulfurization temperatures were set to lower (600 °C) and higher (800 °C) temperatures than the vaporization temperature of MoOx (700 °C), while the second-sulfurization temperature and process time were kept at 1,000 °C and 150 min, respectively (see Methods section). The roughness of the sulfurized MoOx thin films in accordance with first-sulfurization temperature was then compared using SEM and AFM (Fig. 1c–f). Figure 1c,e shows SEM images of the sulfurized MoOx thin films for first-sulfurization temperatures of 600 °C and 800 °C, respectively. The sulfurized MoOx thin film at a first-sulfurization temperature of 600 °C has a smooth and continuous surface, while the MoOx thin film sulfurized at a first-sulfurization temperature of 800 °C has a rough and non-continuous surface. AFM analyses (Fig. 1d,f) illustrate the variations in the roughness of the sulfurized MoOx thin films more clearly, which is due to the differing first-sulfurization temperatures. The r.m.s. value for the MoS2 sulfurized at 600 °C is very low (∼0.4 nm) and is close to the r.m.s. value of the SiO2 substrate (0.37 nm). In contrast, the r.m.s. value of the MoS2 in the 800 °C case is relatively high (0.8 nm). As a result, a first-sulfurization temperature of 600 °C results in MoS2 with uniform and continuous surfaces, due to the fact that the first-sulfurization temperature is lower than the vaporization temperature of MoOx, as we assumed. Based on this result, we used a two-step sulfurization process with a 600 °C first-sulfurization temperature to synthesize continuous MoS2 and Mo1−xWxS2 alloys.
Next, layer-number-controlled MoS2 was synthesized utilizing the two-step sulfurization process described above. Figure 2a–d shows the AFM images and height profiles of the transferred MoS2, which were synthesized by sulfurizing MoOx thin films deposited by 6, 9 and 12 ALD cycles. The measured thicknesses of the synthesized MoS2 were ∼1, 1.6 and 2.3 nm for 6, 9 and 12 MoOx ALD cycles, respectively. These thicknesses correspond to mono-, bi- and tri-layer (1, 2 and 3l) MoS2, considering that the height of 1l MoS2 on SiO2 is ∼1 nm and the spacing between the first and second MoS2 layers is ∼0.6 nm (refs 3, 4). As reported previously, the larger AFM-measured spacing between the first MoS2 layer and the substrate, compared with that between the MoS2 layers, is caused by the effect of distinct tip–sample and tip–substrate interactions3,30,32. Also, the apparent colour gains of the transferred 1, 2 and 3l MoS2 are observed in optical microscopy (OM) images (Supplementary Fig. 2). It should be noted that the MoS2 is not formed in the case of an ALD MoOx thin film with an ALD cycle number of <3 (Supplementary Fig. 3). This is attributed to a nucleation delay during the initial growth of the MoOx, and similar behaviour was observed during the synthesis of WS2 by sulfurization of ALD WO3 (ref. 30). After the nucleation delay, 1l of MoS2 is formed by the sulfurization of each three-cycle ALD MoOx thin film sample (∼0.8−0.9 nm in thickness). This observation agrees with a previous report, where ∼1 nm of MoOx film transformed into a 1l MoS2 via sulfurization33. The stoichiometry calculated from X-ray photoemission spectroscopy (XPS) result is 2 (S/Mo) as shown in Supplementary Fig. 4. As a result, we can systematically control the layer number of MoS2 by controlling the ALD MoOx cycle number.
(a–c) AFM images and (d) height profiles (along with white dashed line in AFM images) of transferred MoS2 on SiO2 substrate for 1l, 2l and 3l thickness, respectively. Scale bars, 0.5 μm. (e) Raman spectra and (f) PL spectra for 1l (red), 2l (blue) and 3l (black) MoS2 on SiO2 substrate. (g) HRTEM image of 1l MoS2 at a selected region and (inset) FFT pattern. Scale bars, 2 nm.
The MoS2 were further characterized using Raman, PL and high-resolution TEM (HRTEM). The Raman spectra (λexc=532 nm) for 1, 2l and 3l MoS2 are shown in Fig. 2e. The MoS2 exhibit in-plane and out-of-plane vibrations modes at 386.6 and 406.5 cm−1 (E′ and A′1) for the 1l, 385.6 and 407.6 cm−1 (Eg1 and A1g) for 2l, and 384.7 and 408.5 cm−1 (E′1 and A′1) for 3l (ref. 34). From the Raman spectra, we calculated the relative peak distance between the in-plane and out-of-plane modes, which is closely related to the layer number of the MoS2 due to the softening in the in-plane and stiffening in the out-of-plane mode frequencies, with increasing layer numbers35,36. The calculated relative peak distances are 19.9, 22 and 23.8 cm−1 for the 1, 2 and 3l samples, respectively, which are in good agreement with previously reported values for synthesized MoS2 (refs 37, 38, 39).
The PL spectra dependence on the layer number of the MoS2 is shown in Fig. 2f. The spectrum of the 1l MoS2 shows PL peaks at 1.89 eV and 2.01 eV, which are correlated to the A1 and B1 direct excitonic transitions of the MoS2, respectively. With increasing layer number, weak PL peaks are observed at 1.87 eV and 2.00 eV for the 2l, and 1.86 eV and 1.99 eV for the 3l. The red shift and low intensity of the PL peaks with increasing layer number is due to the band gap transition from direct to indirect, which is consistent with the dependency of the PL peak on the layer number4,5,6,40. These Raman and PL results again confirm the layer controllability of MoS2 using the ALD process. Figure 2g is an HRTEM image for the synthesized 1l MoS2. The MoS2 shows a honeycomb-like structure with lattice spacing of 0.27 nm and 0.16 nm for the (100) and (110) planes, respectively. In addition, sixfold coordination symmetry is observed in the fast Fourier transformation (FFT) image (inset of Fig. 2g). The approximate domain size is 10−20 nm, similar to that of previously reported synthesized MoS2 and WS2 using the sulfurization of MoOx and WO3 thin films30,41.
Mo1−xWxS2 alloy synthesis
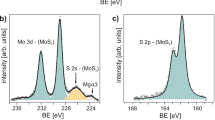
A super-cycle ALD-based Mo1−xWxS2 alloy synthesis process was developed based on the synthesis processes for 2D MoS2 (this study) and WS2 (previous study)30. The overall synthesis scheme for the Mo1−xWxS2 alloy is illustrated in Fig. 3a. First, we conducted 10 cycles of WO3 ALD to address the nucleation delay of the ALD WO3 (ref. 30) (not shown in Fig. 3a). Subsequently, one super-cycle ALD process consisting of n cycles of ALD MoOx and m cycles of ALD WO3 was conducted and the deposited Mo1−xWxOy alloy thin films were sulfurized. We used varying cycles for MoOx (n) and WO3 (m) in one super-cycle to deposit 0.8−0.9-nm-thick composition-controlled Mo1−xWxOy alloy thin films to create a 1l Mo1−xWxS2 alloy. This was based on the growth rate of ALD MoOx (2.7 Å per cycle) and WO3 (0.9 Å per cycle), as shown in Supplementary Table 1. Figure 3b–d shows the XPS spectra of the 1l MoS2, 1l WS2 and sulfurized Mo1−xWxOy alloy thin films with different n and m numbers in one super-cycle. All measured XPS results were normalized by S2p3/2 peak intensity and calibrated to the C1s peak at 285 eV. With increasing n/m ratio, the intensity of the Mo3d peaks increased, while the W5p3/2 and W4f peaks decreased. Furthermore, the peak positions for Mo3d and W4f gradually shifted to higher binding energies, from 232.2 eV and 229.1 eV to 232.5 eV and 229.4 eV for Mo3d3/2 and Mo3d5/2, respectively, and from 34.8 eV and 32.6 eV to 35.0 eV and 32.8 eV for W4f5/2 and W4f7/2, respectively. In addition, the S2p peaks shifted to lower binding energies, from 163.5 eV and 162.4 eV to 163.3 eV and 162.2 eV for S2p1/2 and S2p3/2, respectively. This small shift in peak position is attributed to the enhanced electron attraction strength of S and the reduced electron attraction strength of W, following increased Mo content due to smaller electronegativity of Mo (2.16) than that of W (2.36) as previously reported7. It is noteworthy that the Mo6+ 3d3/2 peak, which is attributed to the Mo–O bonding, is not observed in the Mo3d spectra; this indicates the absence of O species.
We calculated the Mo, W and S concentrations from the XPS results for the Mo3d, W4f and S2p peaks, respectively, to examine the Mo1−xWxS2 alloy composition. Table 1 presents the calculated concentration and W composition, x. The calculated x value is dependent on the n and m numbers in a single super-cycle, and yields x=0.8 for n=1 and m=6, x=0.6 for n=2 and m=4, and x=0.3 for n=3 and m=1. Also, the calculated stoichiometry is 2 (S/(Mo+W)). This shows that the W composition (x) in the Mo1−xWxS2 alloys can be systematically modulated by changing the values of n and m in one super-cycle.
The synthesized composition-controlled Mo1−xWxS2 alloy from super-cycle ALD Mo1−xWxOy alloy thin films were characterized using AFM, Raman and PL, as shown in Fig. 4. The AFM images and height profiles of the transferred Mo1−xWxS2 alloys are represented in Fig. 4a−d and they show good uniformity and continuity (also see OM images in Supplementary Fig. 5). The measured thicknesses of the Mo0.2W0.8S2, Mo0.4W0.6S2 and Mo0.7W0.3S2 alloys were all ∼1 nm, corresponding to the 1l thickness of Mo1−xWxS2 alloy. Furthermore, 2 and 3l Mo1−xWxS2 alloys can be synthesized using two- and three-super-cycle ALD Mo1−xWxOy alloy thin films (Supplementary Fig. 6). As a result, our super-cycle ALD-based Mo1−xWxS2 alloy synthesis process can systematically control the layer number, as well as the composition of the resultant alloys through manipulation of the super-cycle ALD process.
(a–c) AFM images and (d) height profiles (along with white dashed line in AFM images) of transferred 1l Mo1−xWxS2 alloy on SiO2 substrate for x=0.8, 0.6 and 0.3, respectively. Scale bars, 0.5 μm. (e) Raman spectra and (f) PL spectra for 1l Mo1−xWxS2 alloy on SiO2 substrate for x=1, 0.8, 0.6, 0.3 and 0. (g) PL peak position versus W composition (x) graph. Error bars represent s.d. of PL peak position in five-times repeatedly synthesized Mo1−xWxS2 alloy.
Figure 4e shows the Raman spectra of composition-controlled 1l Mo1−xWxS2 alloys. The 1l WS2 (x=1) exhibits first-order modes: out-of-plane (A′1) and in-plane (E′) modes at 417 cm−1 and 357 cm−1, respectively, and a second-order mode: 2LA(M) at 353 cm−1 (ref. 30). The A′1 mode shifts to a lower frequency with decreasing W composition, while the E′ mode related to WS2 does not noticeably shift with the reduction of intensity. In addition, an E′ mode related to MoS2 appear at x=0.8 and shifted to a higher frequency with a reduction in W composition. The specific peak position dependency on W composition is represented in Supplementary Fig. 7, and the W composition dependence of the Raman spectra of the 1l Mo1−xWxS2 alloy is consistent with previous reports7,8,9.
The normalized PL spectra of the composition-controlled 1l Mo1−xWxS2 alloys are shown in Fig. 4f, also the x values versus the average PL peak positions and s.d. of five-times repeatedly synthesized 1l Mo1−xWxS2 alloys are plotted in Fig. 4g. As the value of x increased from 0 to 1, the averaged PL peak position initially decreases from 1.885 to 1.863 eV, and then gradually increases to 2.021 eV. This non-linear PL peak position behaviour with changing x is the so-called ‘bowing effect’, and has also been reported for other semiconducting alloys and exfoliated 1l Mo1−xWxS2 alloys9,42,43. The bowing effect in 1l Mo1−xWxS2 alloy can be described by the Equation (1),

where b is a bowing parameter. After fitting the experimental results as shown in Fig. 4g (red solid curve), a b value of 0.25±0.03 eV was extracted. The extracted b value is comparable to that of the previous experiment (0.25±0.04 eV) and simulation (0.28±0.04 eV) results9. Furthermore, the s.d. of the five-times repeatedly synthesized 1l Mo1−xWxS2 alloys are small within the range of 0.008 to 0.01, which indicates that the process has good reliability in terms of composition control. Thus, the PL result confirms that we modulate the band gap of the Mo1−xWxS2 alloy by reliably controlling the composition. Moreover, the band gap can also be modulated by controlling the layer number (see Supplementary Fig. 8).
Figure 5a is the HRTEM image of the 1l Mo0.4W0.6S2 alloy (x=0.6). The Mo0.4W0.6S2 alloy shows a periodic atomic arrangement with a honeycomb-like structure and sixfold coordination symmetry, similar to the 1l MoS2 shown in Fig. 2f. To distinguish between the W and Mo atoms in the 1l Mo0.4W0.6S2 alloy, we analysed the Mo0.4W0.6S2 alloy using STEM annular dark-field and energy dispersive X-ray spectrometry (EDX). Figure 5b is the STEM-ADF image of the 1l Mo0.4W0.6S2 alloy. Brighter and less bright spots, which correspond to W and Mo atoms, respectively, are clearly resolved in the ADF image, as previously reported44. The calculated Mo/W ratio from the atom count in Fig. 5b is 0.42:0.58, which differs by <5% from the XPS-measured stoichiometry. In addition, the EDX result in Fig. 5c supports the presence of W, Mo and S species in the 1l Mo0.4W0.6S2 alloy. To extract a clear intensity difference between the W and Mo atoms, we performed an inverse FFT by applying a mask to the yellow dashed square region in Fig. 5b. Figure 5d–e shows the inversed FFT image (Fig. 5d) and intensity profile (Fig. 5e) along with the yellow solid line in Fig. 5d. Although S atoms are not distinguishable in our result as a result of the displacement of S atoms at 200 kV operation voltage by the knock-on mechanism45, the W and Mo atoms are clearly observable, confirming that these elements share the metal atom sites44. The preference for Mo or W atoms at the neighbouring sites of W atoms is evaluated by degree of alloying that can be calculated by Equation (2)23,44,
(a) HRTEM image of 1l Mo0.4W0.6S2 alloy at a selected region, and (inset) FFT pattern. Scale bars, 2 nm. (b) STEM-ADF image of 1l Mo0.4W0.6S2 alloy at a selected region and (c) corresponding EDX spectrum. Scale bars, 1 nm. (d) Inverse FFT image with masking applied to yellow dashed square region in b. Scale bars, 1 nm. (e) Intensity profile of yellow solid line in d. (f) Coloured W atoms with light brown, blue, red, dark red, yellow, green and violet for six, five, four, three, two, one and zero number of neighbouring Mo atoms.

where Pobserved is the averaged ratio of number of neighbouring Mo atoms to total neighbouring sites of W atoms, and Prandom is the total ratio of Mo atoms in the examined layer. Figure 5f represented differently coloured W atoms depending on number of neighbouring Mo atoms: light brown, blue, red, dark red, yellow, green and violet for six, five, four, three, two, one and zero number of neighbouring Mo atoms. The calculated degree of alloying is 99%, which indicate that there is no preference for Mo or W atoms at the neighbouring sites of W atoms and a random mixture of our 1l Mo1−xWxS2 alloy.
A VCC Mo1−xWxS2 synthesis
The composition controllability of our ALD-based Mo1−xWxS2 alloy synthesis process enables synthesis of a VCC Mo1−xWxS2 multilayer with a clean interface, strong interlayer coupling and broadband light absorption. We sulfurized a VCC Mo1−xWxOy thin film that was deposited by a sequential super-cycle ALD process, so as to synthesize a VCC Mo1−xWxS2 multilayer, as shown in Fig. 6a. First, we conducted 20 cycles of WO3 ALD on a SiO2 substrate, corresponding to 1l WS2. We immediately performed three super-cycles of Mo1−xWxOy ALD with different super-cycle n and m numbers, in the following order: n=1 and m=6, n=2 and m=4, and n=3 and m=1. Last, we conducted three cycles of MoOx ALD (n=3) corresponding to 1l MoS2. The deposited VCC Mo1−xWxOy thin film was sulfurized to convert it into a VCC Mo1−xWxS2 multilayer. Figure 6b,c shows an AFM image and height profile of the transferred VCC Mo1−xWxS2 multilayer, with a measured thickness of ∼3.5 nm. This thickness, synthesized by five sequential ALD super-cycles, corresponds to a 5l Mo1−xWxS2 alloy, which is consistent with each super-cycle result for the 1l Mo1−xWxS2 alloy.
(a) Sequential super-cycle ALD procedure and schematic structure of a VCC Mo1−xWxS2 multilayer. (b) AFM image and (c) height profiles (along with white dashed line in AFM image) for a VCC Mo1−xWxS2 multilayer. Scale bars, 0.5 μm. (d) Calculated atomic concentration and relative concentration ratio of Mo and W from ARXPS measurement. (e) Raman spectra for a VCC Mo1−xWxS2 multilayer. (f) Raman peak position of A1g and MoS2-like E12g modes from fitted Raman spectra (red and blue solid line) and from measured Raman spectra of 1l Mo1−xWxS2 alloy (black dashed line). (g) Calculated Raman peak distances between A1g and MoS2-like E12g modes from fitted Raman spectra (red solid line) and from measured Raman spectra of 1l Mo1−xWxS2 alloy (black dashed line).
The different composition concentrations of the bottom and top layers in the VCC Mo1−xWxS2 multilayer were analysed using angle-resolved XPS (ARXPS). Figure 6d shows the calculated atomic and relative concentration ratios of the Mo and W from the ARXPS measurement (ARXPS spectra are shown in Supplementary Fig. 9). The Mo concentration increased from 18.6 to 20.9%, while the W concentration decreased from 15.7 to 13.5%, with increasing emission angle from 0 to 70° (red line). The Mo/W concentration ratio increased from 1.17 to 1.55 with increasing emission angle (blue line). Although the exact atomic concentration according to position in the VCC Mo1−xWxS2 multilayer cannot be calculated because of the larger depth resolution of the XPS measurement in comparison with the VCC Mo1−xWxS2 multilayer thickness, the emission angle dependency of the Mo and W concentration indicates Mo-rich and W-rich concentration in the upper and lower layers of the VCC Mo1−xWxS2 multilayer, respectively. As a result, ARXPS shows that the VCC Mo1−xWxS2 multilayer has VCC characteristics. Notably, the calculated stoichiometry ratio was 2 (S/(Mo+W)) in all ARXPS results.
The formation of Mo1−xWxS2 alloy with different compositions in a VCC Mo1−xWxS2 multilayer was analysed using Raman spectroscopy. Figure 6e shows the Raman spectrum of a VCC Mo1−xWxS2 multilayer, which exhibits strong peaks for A1g, MoS2-like E12g and WS2-like E12g+2LA(M) modes. Each Raman peak can be fitted using a Lorentzian function to the Raman spectrum of the Mo1−xWxS2 alloy with x=0, 0.3, 0.6, 0.8 and 1. The fitted Raman spectrum was compared with the measured Raman spectrum for the 1l Mo1−xWxS2 alloy, with respect to variations in the peak position and peak distances of the A1g and MoS2-like E12g modes, depending on W concentration. The A1g and MoS2-like E12g peak positions from the fitted Raman spectrum are represented in Fig. 6f with the measured Raman peak positions for the 1l Mo1−xWxS2 alloy (black dashed line, the same as Supplementary Fig. 7). The variation in the fitted Raman peak position with increasing W concentration in the Mo1−xWxS2 alloy is the same as the variation in the measured Raman peak position for the 1l Mo1−xWxS2 alloy: A1g shifts to a higher frequency with an increase in W concentration, while the MoS2-like E12g modes downshift. Figure 6g shows peak distances between the A1g and MoS2-like E12g modes from the fitted Raman spectrum (red solid line) and measured Raman spectrum of the 1l Mo1−xWxS2 alloy (black dashed line), which are 3−4 cm−1 larger than that of the Raman spectrum of the 1l Mo1−xWxS2 alloy. This is due to the softening in the MoS2-like E12g mode frequency and stiffening in the A1g mode frequency. Similar behaviour, that is, increasing peak distances with increasing layer number, is also observed in MoS2 (refs 35, 36) and WS2 (ref. 30) because of the reduced long-range Coulomb interaction between the effective charges, which is induced by an increase in the dielectric screening. These results for the fitted Raman spectra are in good agreement with the dependency of the peak positions on the W composition given by the measured Raman results, and the dependency of the peak distances on layer number in 2D TMDCs. Thus, we can conclude that the fitted Raman spectra show the formation of a Mo1−xWxS2 alloy with different compositions in a VCC Mo1−xWxS2 multilayer.
As a result, it can be stated that the ARXPS and Raman results show the VCC characteristics of a VCC Mo1−xWxS2 multilayer. Also, these findings indicate that the vertical interdiffusion of the Mo and W atoms during the sulfurization process have no critically effect on the VCC characteristics. A similar result was observed in a previous report, in that MoOx/WO3 thin film was converted to MoS2/WS2 without the formation of a Mo1−xWxS2 alloy, indicating the limited interdiffusion of Mo and W atoms46. Further, it is noteworthy that we verified the validity of ARXPS and Raman measurements as a means of characterizing the VCC Mo1−xWxS2 multilayer via characterization of a VCC Mo1−xWxS2 multilayer synthesized with a reversed vertical composition profile (see Supplementary Fig. 10).
Since the interlayer coupling affects interlayer transition15,21,47,48, strong interlayer coupling in a synthesized VCC Mo1−xWxS2 multilayer was evaluated using comparison of interlayer transition in three difference sample types as shown in Fig. 7a. Sample 1 is a stacked VCC Mo1−xWxS2 multilayer fabricated by the transfer of each differently composed Mo1−xWxS2 alloy onto glass substrate, while sample 2 is the same as sample 1 but annealed at 200 °C for 15 min in an Ar ambient atmosphere to enhance the interlayer coupling by the removal of residual molecules21,48. Sample 3 is a transferred VCC Mo1−xWxS2 multilayer on glass substrate, which was annealed at 200 °C for 15 min in an Ar ambient atmosphere. Ultraviolet–visible spectrophotometer measurements for samples 1, 2 and 3 (Fig. 7b) illustrate that these have broadband light absorption properties due to the sum of the light absorption from the differently composed Mo1−xWxS2 alloys. In previous reports, the absorption spectrum of the interlayer transition could be obtained by comparing the intensity difference between the absorption spectra of the weakly interlayer-coupled sample and that of the strongly interlayer-coupled sample15,48. Based on these reports, we extracted the interlayer transition absorption spectrum by subtracting the absorption spectrum of sample 1 from that of sample 2 and of sample 3, since sample 1 has the weakest interlayer coupling of the three samples as a result of the contamination at the interface caused by the layer transfer process15,21,48. The extracted absorption spectra of the interlayer transition are shown in Fig. 7c. The sample 2–sample 1 spectrum (black solid line) shows a small absorbance peak at 1.87 eV, while the sample 3–sample 1 spectrum (red solid line) shows an absorbance peak that is over five times stronger than the sample 2–sample 1 absorbance peak at the same position. Specific observations on the origin of the absorbance peak position from the interlayer transition (1.87 eV) are described in the Supplementary Information (Supplementary Fig. 11). The stronger absorbance peak of sample 3–sample 1 in comparison with that of sample 2–sample 1 indicates that sample 3 has stronger interlayer coupling compared with sample 2. In other words, a VCC Mo1−xWxS2 multilayer based on sequential super-cycle ALD has the strongest interlayer coupling among the three types of samples. We surmise that this strong interlayer coupling results from the absence of a transfer process, which eliminates the incorporation of residual molecules such as H2O and organic contaminants15,21,47,48.
(a) Schematics of three sample types for ultraviolet–visible spectrophotometer measurement. (b) Absorption spectra of sample 1 (black solid line), sample 2 (blue solid line), and sample 3 (red solid line) and (c) extracted absorption spectra of interlayer transition using subtraction of sample 1 from sample 2 (black solid line) and from sample 3 (red solid line). (d) Spectral and (e) time-resolved photocurrent of a VCC Mo1−xWxS2 multilayer, 5l WS2 and 5l MoS2 photodetectors.
A VCC Mo1−xWxS2 multilayer exhibits a broadband light absorption property, as well as strong interlayer coupling. Thus, the VCC Mo1−xWxS2 multilayer has promising potential use as an active layer in an efficient photodetector. To evaluate the photoinduced response of the VCC Mo1−xWxS2 multilayer, we observed the spectral and time-resolved photocurrent of a VCC Mo1−xWxS2 multilayer photodetector and compared it with 5l WS2 and 5l MoS2 photodetectors (see the Methods section for details of device fabrication, and see Supplementary Fig. 12 for AFM images of the 5l MoS2 and WS2 and their I–V characteristics). Figure 7d shows the dependence of the photocurrent on the illumination energy for the VCC Mo1−xWxS2 multilayer, 5l WS2, and 5l MoS2 photodetectors for a voltage drain to source (Vds) of 5 V. The continuum power spectral density is represented in Supplementary Fig. 13. The VCC Mo1−xWxS2 multilayer photodetector generates a broadband photoinduced current from 1.2 to 2.5 eV, because of its broadband light absorption property. In contrast, the 5l WS2 and 5l MoS2 photodetectors generate narrower photocurrents than the VCC Mo1−xWxS2 multilayer, at 1.3 and 2.1 eV for the 5l WS2 photodetector and 1.2 and 1.8 eV for the MoS2 device; these values correspond to the 5l WS2 and MoS2 bandgaps. We then examined the time-resolved photocurrent measurement using white-light illumination, as shown in Fig. 7e (result using specific laser wavelength is shown in Supplementary Fig. 14). The white light was first turned off for a period of 5 s, and then turned on for 5 s with the biasing Vds=5 V. The drain current (Ids) increased on activation of the light and decayed following removal of the incident light. The induced photocurrents were 39 pA, 13 pA and 11 pA for the VCC Mo1−xWxS2 multilayer, 5l WS2 and 5l MoS2 devices, respectively. Hence, the VCC Mo1−xWxS2 multilayer generates three to four times greater photocurrent than 5l WS2 or 5l MoS2, which is attributed to broadband light absorption. Thus, we concluded that the VCC Mo1−xWxS2 multilayer is promising as regards use as an efficient photodetector with broadband light absorption. Furthermore, the broadband light absorption property is feasible for various optoelectronic applications such as solar cells49,50.
Discussion
In summary, we developed an ALD-based Mo1−xWxS2 synthesis process using sulfurization of super-cycle ALD Mo1−xWxOy thin film. We studied the sulfurization process of ALD MoOx thin films to produce uniform and continuous MoS2. The synthesized ALD-based Mo1−xWxS2 alloy show good stoichiometry, uniform and continuous surfaces, controlled composition and layer numbers, and mixing of Mo and W atoms. Moreover, we developed a simple method to synthesize a VCC Mo1−xWxS2 multilayer with a clean interface, which shows stronger interlayer coupling than that of a stacked VCC Mo1−xWxS2 multilayer fabricated using the transfer process. Further, we have shown that the VCC Mo1−xWxS2 multilayer has promising potential applications as an efficient photodetector, because of its broadband light absorption capability. It should also be noted that the ALD-based TMDCs alloy synthesis process is not only limited to Mo1−xWxS2, and we expect that similar process strategies can be developed for other TMDCs materials and their vertical stacks.
Methods
MoOx film growth and characteristics
A 6-inch ALD chamber containing a loadlock chamber was used for the deposition of the MoOx films. The films were deposited on SiO2(300 nm)/Si substrates by plasma-enhanced ALD using Mo(CO)6 and O2 plasma at a 200 °C growth temperature. The temperature of the bubbler containing Mo(CO)6 was maintained at 35 °C to produce adequate vapour pressure, and vapourized Mo(CO)6 molecules were transported into the chamber by pure argon (99.999%) carrier gas. The O2 flow and plasma power were fixed at 300 s.c.c.m. and 200 W, respectively. An ALD cycle consists of four steps: Mo(CO)6 precursor exposure (ts), Ar purging (tp), O2 plasma reactant exposure (tr) and another Ar purging (tp). In the ALD MoOx process, the ts, tp and tr were fixed at 5 s, 12 s and 5 s, respectively. Optimization of the ALD MoOx process is described in the Supplementary Fig. 1.
Mo1−xWxOy Film Growth
MoOx and WO3 ALD processes27 were used to deposit Mo1−xWxOy film using super-cycle ALD (as shown in Fig. 3a) under the same chamber and deposition conditions described above for the ALD of MoOx. After 10 cycles of WO3 ALD to address nucleation delay27, we conducted super-cycle ALD, which consists of n cycles of MoOx ALD and m cycles of WO3 ALD. The detailed process steps are shown in Supplementary Table 1.
Sulfurization processes
One-step process. To sulfurize the ALD MoOx, the sample was placed in the centre of a tube furnace (1.2 inch in diameter). Initially, the sample was heated at 200 °C for 60 min under flowing H2 (25 s.c.c.m.) and Ar (25 s.c.c.m.) gas, to remove any organic contaminants on the surface. Subsequently, the temperature was gradually increased from 200 to 1,000 °C at 13.3 °C min−1, and this temperature was then maintained for 60 min with flowing Ar (50 s.c.c.m.) and H2S (5 s.c.c.m.). Then, the sample was cooled to room temperature under a flowing Ar (50 s.c.c.m.) atmosphere.
Two-step process. Initially, samples were annealed at 200 °C as in the one-step process. Then, the temperature was gradually increased from 200 °C to first-sulfurization temperatures of 600 or 800 °C at 13.3 °C min−1. The peak temperature (600 or 800 °C) was maintained for 60 min with flowing Ar (50 s.c.c.m.) and H2S (5 s.c.c.m.). Subsequently, the temperature was gradually increased from the first-sulfurization temperatures to 1,000 °C at 13.3 °C min−1 and was maintained at this temperature for 30 min with flowing Ar (50 s.c.c.m.) and H2S (5 s.c.c.m.). The sample was cooled to room temperature under a flowing Ar (50 s.c.c.m.) atmosphere after the process was completed. Based on the results shown in Fig. 1, we used a two-step sulfurization process with a 600 °C first-sulfurization temperature to sulfurize MoOx and Mo1−xWxOy thin films in the MoS2 and Mo1−xWxS2 alloy synthesis process.
Transfer of MoS2 and Mo1−xWxS2
The as-synthesized MoS2 and Mo1−xWxS2 on the SiO2 substrate were coated with polymethyl methacrylate (PMMA) by spin coating at 4,000 r.p.m. for 60 s. After curing of the PMMA at 100 °C for 15 min, the samples were immersed in 10% hydrogen fluoride solution to etch the SiO2 layer. Subsequently, the samples were washed using deionized (DI) water and scooped onto a clean SiO2/Si substrate. The PMMA was removed by acetone and washed away using isopropyl alcohol.
Characterization of MoS2 and Mo1−xWxS2
OM (Olympus DX51), Raman spectroscopy (HORIBA, Lab Ram ARAMIS; 532-nm laser excitation wavelength), AFM (VEECO, Multimode), PL (SPEX1403, SPEX; 532-nm laser excitation wavelength), absorbance with ultraviolet–visible spectrophotometer (JASCO Corporation, V-650), XPS (Thermo UK, K-alpha), SEM (JEOL Ltd, JSM-6701F), TEM (FEI Titan G2 Cube 60-300; accelerating voltage, 80 kV), STEM and EDX (JEM 2100F; accelerating voltage, 200 kV) analyses were employed to characterize the MoS2 and Mo1−xWxS2 alloy, and a VCC Mo1−xWxS2 multilayer.
Fabrication and characterization of photodetectors
Photodetectors were fabricated from an as-synthesized VCC Mo1−xWxS2 multilayer, 5l WS2 and 5l MoS2 on a SiO2 (300 nm)/Si substrate by evaporating Au(40 nm)/Ti(1 nm) electrodes with 100-μm channel length. Electrical measurements were conducted using a Keithley 2400 (Keithley Instruments). The photocurrent was measured by modulating the laser beam with a mechanical chopper (1,000 Hz) and detecting the photocurrent with a current preamplifier and a lock-in amplifier. A monochromator was used for wavelength-dependent measurements of the photocurrent.
Parameters for analysis of XPS and Raman
We used Spectral Data Processor v4.1 for the XPS and Raman spectra fitting. In the fitting analysis of the XPS spectra, the full widths at half maximum (FWHM) were between 1.7 and 1.9 eV, the Lorentzian Gaussian Ratio was 2:8, the energy difference between the Mo3d spin-orbit doublet was set to 3.2 eV and the branching ratio was 2/3. In addition, we used Scofield Relative Sensitivity Factor for calculation of stoichiometry as represented in Supplementary Table 2. For the Raman spectrum fitting analysis, the FWHM was between 7 and 12 cm−1.
Additional information
How to cite this article: Song, J. G. et al. Controllable synthesis of molybdenum tungsten disulfide alloy for vertically composition-controlled multilayer. Nat. Commun. 6:7817 doi: 10.1038/ncomms8817 (2015).
References
Bernardi, M. et al. Extraordinary sunlight absorption and 1 nm-thick photovoltaics using two-dimensional monolayer materials. Nano Lett. 13, 3664–3670 (2013).
Wang, Q. H. et al. Electronics and optoelectronics of two-dimensional transition metal dichalcogenides. Nat. Nanotechnol. 7, 699–712 (2012).
Chhowalla, M. et al. The chemistry of two-dimensional layered transition metal dichalcogenide nanosheets. Nat. Chem. 5, 263–275 (2013).
Eda, G. et al. Photoluminescence from chemically exfoliated MoS2 . Nano Lett. 11, 5111–5116 (2011).
Mak, K. et al. Atomically thin MoS2: a new direct-gap semiconductor. Phys. Rev. Lett. 105, 136805 (2010).
Lee, H. S. et al. MoS2 nanosheet phototransistors with thickness-modulated optical energy gap. Nano Lett. 12, 3695–3700 (2012).
Liu, H. et al. Vapor-phase growth and characterization of Mo1-xWxS2 (0≤x≤1) atomic layers on 2-inch sapphire substrates. Nanoscale 6, 624–629 (2014).
Chen, Y. et al. Composition-dependent raman modes of Mo1-xWxS2 monolayer alloys.. Nanoscale 6, 2833–2839 (2014).
Chen, Y. et al. Tunable band gap photoluminescence from atomically thin transition-metal dichalcogenide alloys. ACS Nano 7, 4610–4616 (2013).
Wei, X.-L. et al. Modulating the atomic and electronic structures through alloying and heterostructure of single-layer MoS2 . J. Mater. Chem. A 2, 2101–2109 (2014).
Feng, Q. et al. Growth of large-area 2D MoS2(1-x)Se2x semiconductor alloys. Adv. Mater. 26, 2648–2653 (2014).
Xi, J. et al. Tunable electronic properties of two-dimensional transition metal dichalcogenide alloys: a first-principles prediction. J. Phys. Chem. Lett. 5, 285–291 (2013).
Lin, Z. et al. Facile synthesis of MoS2 and MoxW1-xS2 triangular monolayers. APL Mater. 2, 092514 (2014).
Tannous, J. et al. Synthesis and tribological performance of novel MoxW1−xS2 (0 ≤ x ≤ 1) inorganic fullerenes. Tribol. Lett. 37, 83–92 (2010).
Yu, Y. et al. Equally efficient interlayer exciton relaxation and improved absorption in epitaxial and non-epitaxial MoS2/WS2 heterostructures. Nano Lett. 15, 486–491 (2015).
Hong, X. et al. Ultrafast charge transfer in atomically thin MoS2/WS2 heterostructures. Nat. Nanotechnol. 9, 682–686 (2014).
Kośmider, K. et al. Electronic properties of the MoS2-WS2 heterojunction. Phys. Rev. B 87, 075451 (2013).
Huo, N. et al. Novel and enhanced optoelectronic performances of multilayer MoS2–WS2 heterostructure transistors. Adv. Funct. Mater. 24, 7025–7031 (2014).
Jung, Y. et al. Chemically synthesized heterostructures of two-dimensional molybdenum/tungsten-based dichalcogenides with vertically aligned layers. ACS Nano 8, 9550–9557 (2014).
Cheng, R. et al. Electroluminescence and photocurrent generation from atomically sharp WSe2/MoS2 heterojunctionpn diodes. Nano Lett. 14, 5590–5597 (2014).
Tongay, S. et al. Tuning interlayer coupling in large-area heterostructures with CVD-grown MoS2 and WS2 monolayers. Nano Lett. 14, 3185–3190 (2014).
Terrones, H., López-Urías, F. & Terrones, M. Novel hetero-layered materials with tunable direct band gaps by sandwiching different metal disulfides and diselenides. Sci. Rep. 3, 1549 (2013).
Gong, Y. et al. Band gap engineering and layer-by-layer mapping of selenium-doped molybdenum disulfide. Nano Lett. 14, 442–449 (2013).
Hyungjun, K. Characteristics and applications of plasma enhanced-atomic layer deposition. Thin Solid Films 519, 6639–6644 (2011).
Kim, H. et al. Applications of atomic layer deposition to nanofabrication and emerging nanodevices. Thin Solid Films 517, 2563–2580 (2009).
Lee, J.-S. et al. Atomic layer deposition of Y2O3 and yttrium-doped HfO2 using a newly synthesized Y(iPrCp)2(N-iPr-amd) precursor for a high permittivity gate dielectric. Appl. Surf. Sci. 297, 16–21 (2014).
Nam, T. et al. Growth characteristics and properties of Ga-doped ZnO(GZO) thin films grown by thermal and plasma-enhanced atomic layer deposition. Appl. Surf. Sci. 295, 260–265 (2014).
Kim, W. H. et al. Significant enhancement of the dielectric constant through the doping of CeO2 into HfO2 by atomic layer deposition. J. Am. Ceram. Soc. 97, 1164–1169 (2014).
Brahim, C. et al. ZrO2–In2O3 thin layers with gradual ionic to electronic composition synthesized by atomic layer deposition for SOFC applications. J. Mater. Chem. 19, 760–766 (2009).
Song, J.-G. et al. Layer-controlled, wafer-scale, and conformal synthesis of tungsten disulfide nanosheets using atomic layer deposition. ACS Nano 7, 11333–11340 (2013).
Blackburn, P. E. et al. The vaporization of molybdenum and tungsten oxides. J. Phys. Chem. 62, 769–773 (1958).
Gutiérrez, H. R. et al. Extraordinary room-temperature photoluminescence in triangular WS2 monolayers. Nano Lett. 13, 3447–3454 (2013).
Lin, Y.-C. et al. Wafer-scale MoS2 thin layers prepared by MoO3 sulfurization. Nanoscale 4, 6637–6641 (2012).
Terrones, H. et al. New first order Raman-active modes in few layered transition metal dichalcogenides. Sci. Rep. 4, 4215 (2014).
Li, S.-L. et al. Quantitative raman spectrum and reliable thickness identification for atomic layers on insulating substrates. ACS Nano 6, 7381–7388 (2012).
Li, H. et al. From bulk to monolayer MoS2: evolution of Raman scattering. Adv. Funct. Mater. 22, 1385–1390 (2012).
Ling, X. et al. Role of the seeding promoter in MoS2 growth by chemical vapor deposition. Nano Lett. 14, 464–472 (2014).
Schmidt, H. et al. Transport properties of monolayer MoS2 grown by chemical vapor deposition. Nano Lett. 14, 1909–1913 (2014).
Yu, Y. et al. Controlled scalable synthesis of uniform, high-quality monolayer and few-layer MoS2 films. Sci. Rep. 3, 1866 (2013).
Splendiani, A. et al. Emerging photoluminescence in monolayer MoS2. Nano Lett. 10, 1271–1275 (2010).
Lee, Y. et al. Synthesis of wafer-scale uniform molybdenum disulfide films with control over the layer number using a gas phase sulfur precursor. Nanoscale 6, 2821–2826 (2014).
Van Vechten, J. et al. Electronic structures of semiconductor alloys. Phys. Rev. B 1, 3351 (1970).
Yin, W.-J. et al. Origin of the unusually large band-gap bowing and the breakdown of the band-edge distribution rule in the SnxGe1− x alloys. Phys. Rev. B 78, 161203 (2008).
Dumcenco, D. O. et al. Visualization and quantification of transition metal atomic mixing in Mo1− xWxS2 single layers. Nat. Commun. 4, 1351 (2013).
Garcia, A. et al. Analysis of electron beam damage of exfoliated MoS2 sheets and quantitative HAADF-STEM imaging. Ultramicroscopy 146, 33–38 (2014).
Elías, A. L. et al. Controlled synthesis and transfer of large-area WS2 sheets: from single layer to few layers. ACS Nano 7, 5235–5242 (2013).
Gong, Y. et al. Vertical and in-plane heterostructures from WS2/MoS2 monolayers. Nat. Mater. 13, 1135–1142 (2014).
Chiu, M.-H. et al. Spectroscopic signatures for interlayer coupling in MoS2-WSe2 van der Waals stacking. ACS Nano 8, 9649–9656 (2014).
Lin, Q. et al. Efficient light absorption with integrated nanopillar/nanowell arrays for three-dimensional thin-film photovoltaic applications. ACS Nano 7, 2725–2732 (2013).
Aydin, K. et al. Broadband polarization-independent resonant light absorption using ultrathin plasmonic super absorbers. Nat. Commun. 2, 517 (2011).
Acknowledgements
This work was supported by the Center for Integrated Smart Sensors funded by the Ministry of Science, ICT & Future Planning as Global Frontier Project (CISS-2011-0031848), the National Research Foundation of Korea(NRF) grant funded by the Korea government(MSIP) (No. NRF-2014R1A2A1A11052588 and 2011-0013255), and Korea Evaluation Institute of Industrial Technology (KEIT) funded by the Ministry of Trade, Industry and Energy (MOTIE) (Project No. 10050296, Large scale (Over 8) synthesis and evaluation technology of 2D chalcogenides for next generation electronic devices).
Author information
Authors and Affiliations
Contributions
J.-G.S. carried out most experiments and analysed the data. G.H.R. and Z.L. contributed to TEM measurement. S.J.L. and J.-M.M. contributed to PL measurement. S.W.S and H.C contributed to photoresponse measurement. C.W.L. contributed to XPS measurement. T.C., H.J. and Y.K. contributed to Raman and AFM measurement. C.D. and C.L.-M. contributed to development and handling of ALD precursor. J.-G.S., J.P. and H.K. designed the experiment and co-wrote the paper. All of the authors discussed the results and commented on the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Figures 1-14, Supplementary Tables 1-2 and Supplementary References (PDF 1309 kb)
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Song, JG., Ryu, G., Lee, S. et al. Controllable synthesis of molybdenum tungsten disulfide alloy for vertically composition-controlled multilayer. Nat Commun 6, 7817 (2015). https://doi.org/10.1038/ncomms8817
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/ncomms8817
This article is cited by
-
Atomically Substitutional Engineering of Transition Metal Dichalcogenide Layers for Enhancing Tailored Properties and Superior Applications
Nano-Micro Letters (2024)
-
Chemiresistive gas sensor based on Mo0.5W0.5S2 alloy nanoparticles with good selectivity and ppb-level limit of detection to ammonia
Microchimica Acta (2023)
-
Large-area epitaxial growth of 2D ZrS2(1−x)Se2x semiconductor alloys with fully tunable compositions and bandgaps for optoelectronics
Science China Materials (2023)
-
Chemical vapor deposition synthesis of V-doped MoS2
Rare Metals (2023)
-
Uniaxial strain engineered MoS2 (molybdenite) and chlorine adsorbed MoS2 nanostructures for tuning their electronic and optical properties
Optical and Quantum Electronics (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.