Research
Prof. Zonghoon Lee’s Atomic-Scale Electron Microscopy Lab
Prof. Zonghoon Lee’s Atomic-Scale Electron Microscopy Lab
Abstract
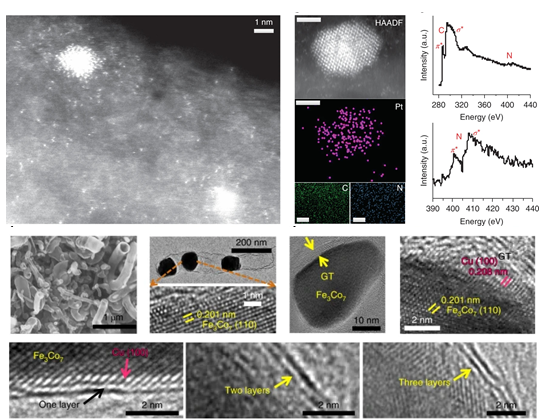
Platinum is the most effective electrocatalyst for the hydrogen evolution reaction in acidic solutions, but its high cost limits its wide application. Therefore, it is desirable to design catalysts that only require minimal amounts of Pt to function, but that are still highly active. Here we report hydrogen production in acidic water using a multicomponent catalyst with an ultralow Pt loading (1.4 μg per electrode area (cm2)) supported on melamine-derived graphitic tubes (GTs) that encapsulate a FeCo alloy and have Cu deposited on the inside tube walls. With a 1/80th Pt loading of a commercial 20% Pt/C catalyst, in 0.5 M H2SO4 the catalyst achieves a current density of 10 mA cm-2 at an overpotential of 18 mV, and shows a turnover frequency of 7.22 s-1 (96 times higher than that of the Pt/C catalyst) and long-term durability (10,000 cycles). We propose that a synergistic effect between the Pt clusters and single Pt atoms embedded in the GTs enhances the catalytic activity.
Our research focuses on atomic-scale characterization, design, and synthesis as well as the properties of advanced materials including 2D materials, carbon materials, and soft matter by means of aberration-corrected transmission electron microscopy and spectroscopy. In situ experiments at both the atomic and nano scales are implemented for our study.
Advanced TEM Characterization